|
|
One-Step Troponin I (cTnl) Rapid Test Uncut Sheet
|
Product Details:
Payment & Shipping Terms:
|
| Features: | Adjustable Temperature, Non-stick Coating, Removable Plates | Spsecificity: | 100% |
|---|---|---|---|
| Cooling Way: | Water Cooling | Usage: | Home Appliance |
| Customization: | Yes | Safety Standard: | Other |
| Car Fitment: | Universal | Products Status: | Stock |
| Monofilament Style: | Yes | Machinery Test Report: | Provided |
| Ice Size: | 10/15/20/25/50/100kg |
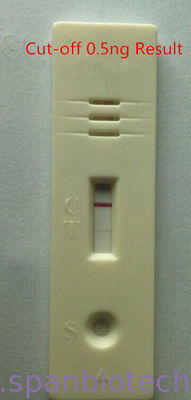
One-Step Troponin I Rapid Test
Package: 30 sheets per pouch
INTENDED USE
ONE STEP TROPONIN I TEST IS AN IMMUNOCHROMATOGRAPHY BASED ONE STEP IN VITRO TEST. IT IS DESIGNED FOR QUALITATIVE DETERMINATION OF CARDIAC TROPONIN I (CTNL) IN HUMAN WHOLE BLOOD, SERUM OR PLASMA AS AN AID IN THE DIAGNOSIS OF MYOCARDIAL INFARCTION.
SUMMARY
Cardiac Troponin I (cTnI) is a cardiac muscle protein with a molecular weight of 22.5 kilodaltons. Together with troponin T (TnT) and troponin C (TnC), TnI forms a troponin complex in heart to play a fundamental role in the transmission of intracellular calcium signal actin-myosin interaction. Although troponin I is also found in skeletal muscle, cardiac troponin I (cTnI) has an additional amino acid residues on its N-terminal which distinguishes it from its skeletal muscle form making cTnI a specific marker for indicating cardiac infarction. cTnI is released rapidly into blood stream soon after the onset of acute myocardial infarction (AMI). Its release pattern is similar to CK-MB (4-6 hours after the onset of AMI). However, CK-MB level returns to normal after 36-48 hours, when levels of cTnI remains elevated for up to 6-10 days. The level of cTnI is below 0.06ng/ml in average in healthy people, and also not detected in the patients with skeletal muscle injury. Therefore, cTnI is a specific marker for diagnosis of AMI patients. The level of cTnI may reach 100-1300ng/ml in some AMI patients.
PRINCIPLE OF THE ASSAY
The One-Step Troponin I Test is a chromatographic immunoassay for the qualitative determination of cTnI in human whole blood, serum or plasma. When specimen is added to sample pad, it moves through the conjugate pad and mobilizes gold anti-cTnI conjugate that is coated on the conjugate pad. The mixture moves along the membrane by capillary action and reacts with anti-cTnI antibody that is coated on the test region. If cTnI is present, the result is the formation of a colored band in the test region. If there is no cTnI in the sample the area will remain colorless. The sample continues to move to the control area and forms a pink color, indicating the test is working and the result is valid.
STORAGE AND STABILITY
1. Store as packaged in the sealed pouch at 2-30°C
2. The test device must remain in the sealed pouch until use.
3. DO NOT FREEZE.
4. Do not use beyond the expiration date.
PRECAUTIONS
1. For professional and IN VITRO diagnostic use only.
2. The test device should remain in the sealed pouch until use. Do not use after the expiration date.
3. All serum or plasma specimens should be considered potentially hazardous and handled in the same manner as an infectious agent.
4. The test device should be discarded in a proper biohazard container after testing.
5. Avoid cross-contamination of serum samples by changing a new specimen pipette for each sample.
SPECIMEN COLLECTION
1. Testing should be performed immediately after the specimens have been collected. Do not leave the specimens at room temperature for prolonged periods. Specimens may be stored at 2-8°C for up to 3 days. For long-term storage, specimens should be kept below -20°C.
2. Bring specimens to room temperature prior to testing. Frozen specimens must be completely thawed and mixed well prior to testing. Specimens should not be frozen and thawed repeatedly.
MATERIALS PROVIDED
1. Test cards individually foil pouched with a desiccant
2. Plastic dropper
3. Package insert
MATERIALS REQUIRED BUT NOT PROVIDED
1. Timer/clock
2. Pipette
3. Controls
Contact Rebecca Yan
Tel: +86(755)89589611
Fax: +86(755)89580096
Cell Phone:+8613417551798(WhatsApp)
Web: www.spanbio.com
Email: rebecca@spanbio.com
Contact Person: Ms. Anna Lee
Tel: +86-755-89589611
Fax: 86-755-89580096