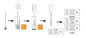|
|
CPV + CCV + Giardia Ag Triple Test
|
Product Details:
Payment & Shipping Terms:
|
| Packagae: | 10 Test/box | Specificity: | 100% |
|---|---|---|---|
| Sensitvity: | 100% | Storage Conditions: | 2-30°C |
| Usage: | In Vitro Diagnostic Use | Accuracy: | >95% |
| Certifications: | CE, ISO 13485 | Type: | Diagnostic Test |
| Country Of Origin: | Varies Depending On Specific Test | Storage: | 2-30℃ |
| Target Analytes: | Various Diseases And Conditions | Sensitivity: | High |
| Product Type: | Rapid Test | Application: | Rapid Detection Of Diseases |
| Package: | 40 Tests/box | Test Type: | Rapid |
| Species: | Dogs |
CPV + CCV + Giardia Ag Triple Test
- INTENDED USE
CPV + CCV +Giardia Ag Triple Test is a combined cassette to differentially diagnose the presence of Canine Parvovirus antigen, Canine Coronavirus antigen and Giardia antigen in dog’s feces or vomit.
Assay Time: 5-10 min
Sample: Feces or vomit
- PRINCIPLE OF THE ASSAY
CPV + CCV + Giardia Ag Triple Test is based on sandwich lateral flow immunochromatographic assay. The test device has three testing windows. Each testing window has an invisible T (test) zone and C (control) zone. When sample is applied into the sample hole on the device, the liquid will laterally flow on the surface of the test strip. If there is enough CPV antigen, CCV antigen or Giardia antigen in the sample, a visible T band will appear in the corresponding testing window. The C band should always appear after a sample is applied, indicating a valid result. By this means, the device can accurately indicate the presence of CPV, CCV or Giardia antigen in the sample.
- KIT COMPONENT
- 10×foil pouches, each containing onecassette and a desiccant
- 10×assay buffer tubes (1.0 mL each)
- 10×pipettes
- 10×swab sticks
- Product Manual
- TEST PROCEDURE
![]()
- Collect dog’s feces or vomit with the swab stick from dog’s cloaca or on the ground.
- Insert the wet swab into the provided assay buffer tube. Agitate it to assure good sample extraction.
- Take out the cassette from the foil pouch and place it horizontally.
- Gradually drip 3 drops each of sample extraction into the sample holes.
- Interpret the result in 5-10 minutes. Result after 10 minutes is considered as reference.
- INTERPRETATION OF RESULTS
Positive: The presence of both “C” band and “T”band, no matter T band is clear or vague.
Negative: Only clear C band appears.
Invalid: No colored band appears in C zone, no matter whether T band appears.
![]()
- STORAGE
The kit can be stored at room temperature (2-30°C). The test kit is stable through the expiration date (18 months) marked on the foilpouch. DO NOT FREEZE. Do not store the test kit in direct sunlight.
- PRECAUTIONS
- For best results, please strictly adhere to these instructions.
- All reagents must be at room temperature before running the assay.
- Do not remove test cassette from its pouch until immediately before use.
- Do not reuse the test kit.
- Do not use the test beyond its expiration date marked on the foil pouch.
- The components in this kit have been quality control tested as standard batch unit. Do not mix components from different lot numbers.
- LIMITATION
CPV + CCV + Giardia Ag Triple Test is for in vitro veterinary diagnosis use only. All results should be considered with other clinical information available from veterinarian. For an accurate result, It is suggested to apply other method such as PCR for final determination in practice.
Span Biotech Ltd. is a research based company for rapid tests, with strong support from National Key Laboratory of Technology Projects of 10th and 11th Five-Year Plan and Faculty of Life Sciences of HuBei University. SpanBio also housed a R&D team that is developing gene recombination, cell cultivation and protein purification techniques. SpanBio pays strict attention on rapid tests for human being, animal diseases and food safety detection. It provides a number of customized services to professional distributors and partnering affiliates with excellent quality, competitive prices and super service.
Our mission:
- Always best of all and always pay attention to innovation.
- Special customized service tightly following customers’ requests.
- Integrated excellent quality, competitive prices and super service together.
Rebecca Yan
Span Biotech Ltd.
Tel: +86(755)89589611
Cell Phone:+8618823462100(WhatsApp)
Web:www.spanbio.com
Contact Person: Ms. Anna Lee
Tel: +86-755-89589611
Fax: 86-755-89580096